
Editor’s note: “Quantum Computing Revolution: The Gargantuan Opportunity Investors Shouldn’t Ignore” was previously published in November 2024 with the title, “Quantum Computing: the Key to Unlocking AI’s Full Potential?” It has since been updated to include the most relevant information available.
For the past two years, AI stocks have been all the rage on Wall Street, regularly outperforming the broader market.
Exchange-traded funds – such as the Global X Artificial Intelligence & Technology ETF (AIQ) and the ARK Autonomous Technology & Robotics ETF (ARKQ) – represent strong proxies for the industry as a whole. And as you can see in the graph below, those AI plays have been killing it.

But recently, a new evolution of AI stocks has emerged as, potentially, this whole AI Revolution’s biggest winners.
They’re called “QAI,” or Quantum Artificial Intelligence, stocks. These are trades at the intersection of quantum computing and artificial intelligence. And they have absolutely soared over recent months.
One is up more than 400% since early September 2024, while another has popped more than 500%. And those are the “small” winners…
Three other QAI stocks have rocketed 1,000% in that same time – with one up more than 3,500%!

These companies are creating the next-gen quantum computers that could entirely revolutionize AI software development and create a new class of super-powered AI applications. And some may just end up being the biggest market winners of this decade.
To understand why, we’ll need to take a deep look into this groundbreaking technology.
What Is Quantum Computing?
Let me start by saying that the underlying physics of this technological breakthrough – quantum mechanics – is a highly complex topic. It would likely require over 500 pages to fully understand.
But, alas, here’s my best job at making a Cliff’s Notes version in 500 words instead.
For centuries, scientists have developed, tested, and validated the laws of the physical world, known as classical mechanics. These scientifically explain how and why things work, where they come from, so on and so forth.
But in 1897, J.J. Thomson discovered the electron. And he unveiled a new, subatomic world of super-small things that didn’t obey the laws of classical mechanics… at all. Instead, they obeyed their own set of rules, which have since become known as quantum mechanics.
The rules of quantum mechanics differ from that of classical mechanics in two very weird, almost-magical ways.
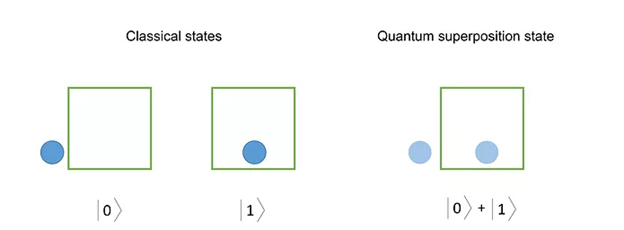
First, in classical mechanics, objects are in one place at one time. You are either at the store or at home, not both.
But in quantum mechanics, subatomic particles can theoretically exist in multiple places at once before they’re observed. A single subatomic particle can exist in point A and point B at the same time until we observe it. And at that point, it only exists at either point A or point B.
So, the true “location” of a subatomic particle is some combination of all its possible positions.
This is called quantum superposition.
Entanglement
Second, in classical mechanics, objects can only “work” with things that are also “real.” Of course, you can’t use an imaginary friend to help move the couch. You need a real friend instead.
But remember how the true location of a subatomic particle is the combination of all of its probabilistic states? Well, all those states are not independent; they’re entangled. So, if we know something about the probabilistic positioning of one subatomic particle, then we know something about the probabilistic positioning of another. It’s all connected. And that means that theoretically, all of these probabilistic states can work together, all at once, to create a super-complex ecosystem.
This is called quantum entanglement.
Between entanglement and superpositioning, subatomic particles can theoretically have multiple probabilistic states at once. And all those states can work together – again, all at once – to accomplish some task.
Pretty wild, right?
It goes against everything classical mechanics taught us about the world. It goes against common sense. But it’s true. It’s real. And now, for the first time ever, we are learning how to harness this unique phenomenon to change everything about everything…
That is why some folks think quantum computing could be more revolutionary for humankind than the discovery of fire or the invention of the wheel.
And I agree.
Mark my words. Quantum mechanics could very well reshape our world over the next few years. And some investors may end up making a lot of money because of it.
Quantum Computing Will Change the World
You see; the study of quantum theory has made huge advancements over the past century, especially so over the past decade.
Scientists at leading laboratories and tech companies have started figuring out how to harness the almost-magical powers of quantum mechanics to make a new generation of super quantum computers. These devices are infinitely faster and more powerful than even today’s fastest supercomputers.
In the words of Haim Israel, Bank of America’s head of Thematic Research:
“By the end of this decade, the amount of calculations that we can make [on a quantum computer] will be more than the atoms in the visible universe.”
Again, the physics behind quantum computers is highly complex. But here’s my shortened version…
Today’s computers are built on top of the laws of classical mechanics. That is, they store information on what are called bits, which can store data binarily as either “1” or “0.”
But what if you could turn those classical bits into quantum bits – qubits – to leverage superpositioning to be both “1” and “0” stores at once?
Further, what if you could leverage entanglement and have all multi-state qubits work together to solve computationally taxing problems?
Theoretically, you’d create a machine with so much computational power that it would make today’s most advanced supercomputers seem ancient.
That’s exactly what’s happening today.
The Possibilities Behind Quantum Computing
Earlier this month, Google unveiled its latest quantum processor, Willow.
At that reveal, Google announced that Willow had solved a complex calculation – one that takes its fastest classical supercomputer around 10 septillion years to complete – in just five minutes.
Ten septillion years (which, written out, is 10,000,000,000,000,000,000,000,000) cut down to five minutes…
That is the very real power of quantum computers.
Just imagine the possibilities if we could shorten and simplify all the world’s problems in a similar manner.
We may finally have the level of AI that you see in movies. Arguably the biggest limitation to today’s AI is the robustness of machine learning algorithms, which are constrained by supercomputing capacity. Expand that capacity, and you’d get infinitely improved machine learning algos – and infinitely smarter AI.
We may be able to eradicate disease. Of course, we already have tools like gene editing. But, as with AI, gene editing tech’s effectiveness relies on the robustness of the underlying computing capacity to identify, target, insert, cut, or repair genes. With quantum computing capacity, all that could happen without error in seconds.
What about a million-mile EV? We can only improve batteries if we can test them. And we can only test in the real world so much. Therefore, one way to unlock a million-mile battery is through simulation. And the higher the underlying computing capacity, the faster and more effective the simulations.
There’s seemingly no limit to what such powerful computing capacity could lead us to…
Which means the economic opportunities here are truly enormous.
The Final Word
That is why I state with such confidence that quantum computing is the most underrated and most transformational technological breakthrough since the internet.
In fact, it may be bigger than the internet. And Wall Street is starting to take notice.
Per my research, there are five noteworthy pure QAI stocks. All five are up more than 5X since early September. Three have surged more than 10X: Quantum Computing (QUBT), Rigetti (RGTI), and D-Wave Quantum (QBTS). And one – QUBT – has rocketed an astounding 35X.
These stocks are on fire.
Though, to be sure, not all of them will succeed in the long run. Some of these QAI stocks will be flashes in the pan, like how Pets.com or Webvan were back in the 1990s.
But some could easily become the next Nvidia (NVDA) or Microsoft (MSFT).
And that’s why, at the very least, you should pay close attention to developments in the quantum computing industry.
We’re definitely watching this industry like hawks, following every technical development and the stocks that seem to hold the most promise.
If you want to stick with – and profit from – these game-changing QAI stocks, I think we’re the best suited to help you do just that.
Check out our research services today to learn more.
On the date of publication, Luke Lango did not have (either directly or indirectly) any positions in the securities mentioned in this article.
P.S. You can stay up to speed with Luke’s latest market analysis by reading our Daily Notes! Check out the latest issue on your Innovation Investor or Early Stage Investor subscriber site.





